Download a printable version of this document here.
If you want to learn about assigning NMR spectra, check out this page.

NMR stands for nuclear magnetic resonance, and it is exactly that; we are looking at how the nuclei of atoms (made up of protons and neutrons) interact with a magnetic field, and how they resonate (or transition) between energy levels when we fire low-energy radio waves at them. Organic chemists use NMR spectroscopy all the time whilst synthesising new molecules, in order to identify whether reactions have been successful, and what they have made. In fact it is fair to say that NMR spectroscopy has been the single largest factor contributing to the rapid progress in organic chemistry over the last century; before NMR all organic structures had to be determined by a combination of chemical tests and degradation techniques.
Magnetic Resonance
NMR spectroscopy involves the excitation of nuclei by electromagnetic radiation in the radio-frequency range of the electromagnetic spectrum. For a nucleus to absorb energy from radio waves in this way, it must have the quantum mechanical property of spin. Felix Bloch and Edward Purcell discovered the magnetic resonance phenomenon independently in 1946, and went on to win the Nobel Prize for Physics in 1952. This is a physical phenomenon that occurs with the nuclei of atoms that have an odd number of protons and/or an odd number of neutrons:
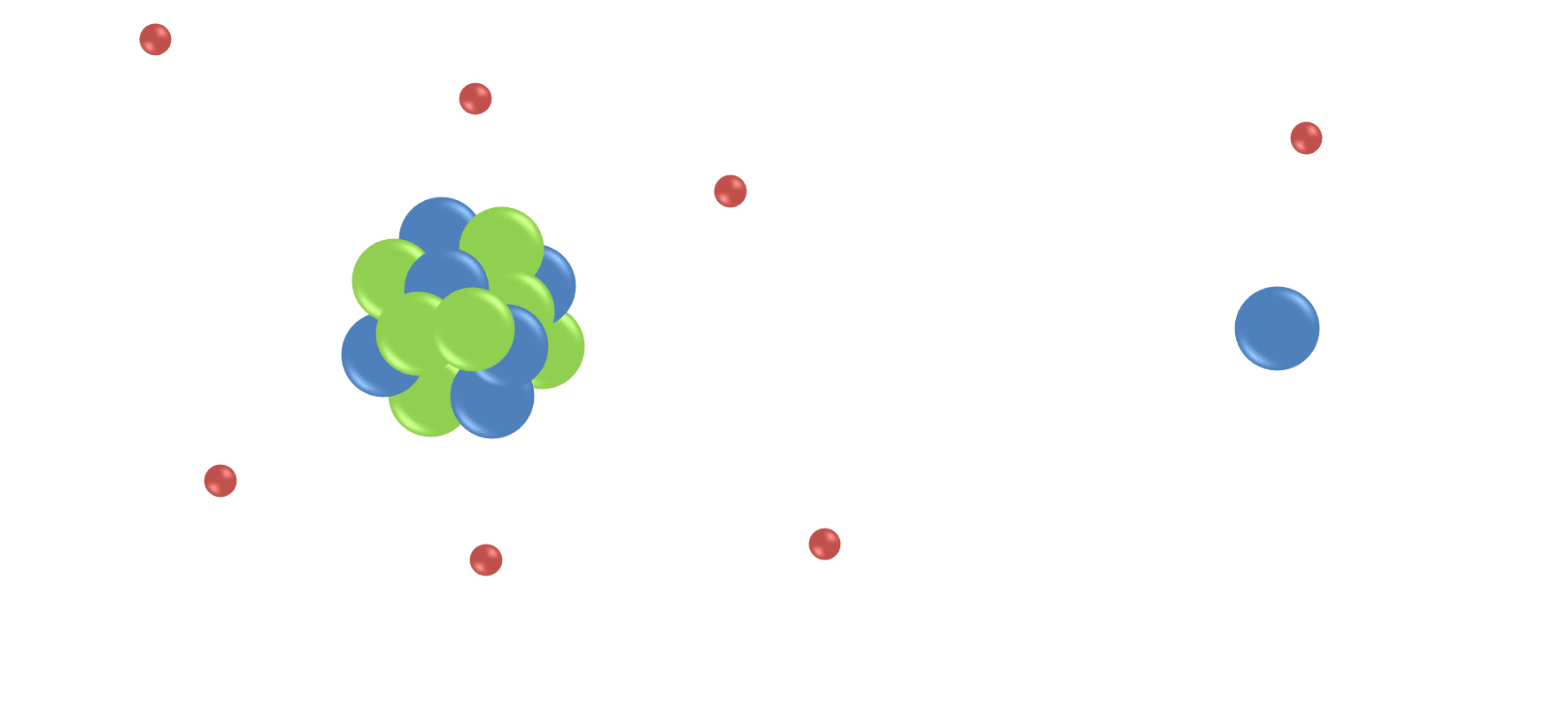
The protons and neutrons within an atom do not actually behave like tiny ping-pong balls, as the image above suggests, but in fact they are more like tiny gyroscopes that spin about their axes in random directions, and generate their own magnetic fields:
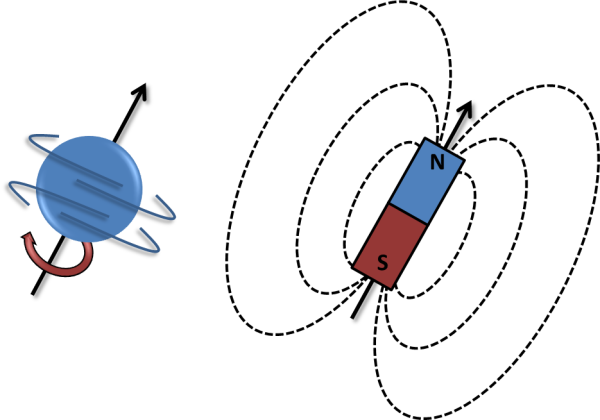
When there are pairs of ‘spinning’ particles, the spins can cancel each other out. However, any nucleus with an odd number of protons and/or neutrons has ‘spin’ due to the overall spin of the unpaired proton or neutron, and therefore generates a tiny magnetic field for the nucleus. Therefore if a nucleus has spin, it can be detected by NMR, because it interacts with magnetic fields.
NMR Spectroscopy
 There are two main types of NMR we use; proton (hydrogen) and carbon. This is very useful for organic molecules, due to the large number of C-H bonds.
There are two main types of NMR we use; proton (hydrogen) and carbon. This is very useful for organic molecules, due to the large number of C-H bonds.
We call 1H NMR proton NMR, because the nucleus of a hydrogen atom is, in fact, just a proton. For carbon NMR, we need to use the isotope 13C, as 12C nuclei do not have spin. This is because they have 6 protons and 6 neutrons. 13C, with 6 protons and 7 neutrons, can be detected by NMR. As only 1 in 100 carbon atoms are 13C, these spectra are quite weak. It is also quite common to look at 31P and 19F NMR, as these atoms also have spin, are quite easy to detect, and are regularly found in organic molecules.
The physical theory that describes the NMR phenomenon is quite complex. In order to understand the basics at a level useful for organic chemistry, consider the following idea: A proton behaves a bit like a tiny bar magnet, or a magnetic dipole, that has two opposite poles; a north and a south. Without an external magnetic field the magnetic dipoles of the protons are all randomly orientated. When protons are placed within a magnetic field, however, its energy obviously depends upon its orientation; i.e. if you take two magnets they prefer to align in the same direction. At the microscopic level of the nuclei, there are only 2 possible energy states, as energy is quantised at the microscopic level; they can align either with the field, or against the field; these two states now are not equivalent, as it takes more energy to be aligned the wrong way around. If we irradiate the sample with low-energy radio waves of the right frequency, the protons can absorb energy to transition between the energy levels; protons in the lower (aligned) state absorb radio energy and jump up to the higher energy state, aligned against the field.
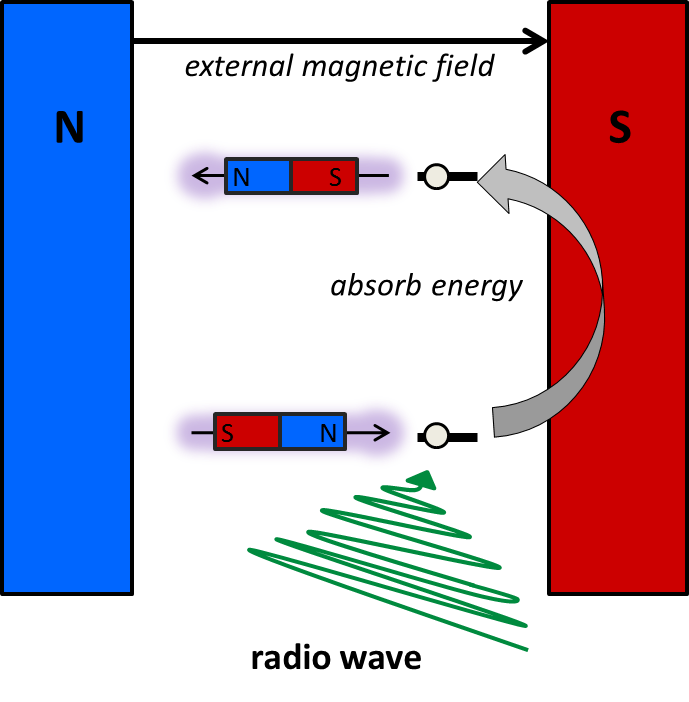 The frequency of the radio wave has to match the energy gap, and this energy gap depends upon the chemical environment of the proton. Therefore, we can use this information to determine the different protons within an organic molecule.
The frequency of the radio wave has to match the energy gap, and this energy gap depends upon the chemical environment of the proton. Therefore, we can use this information to determine the different protons within an organic molecule.
Conventional NMR spectrometers do not work like this anymore, but originally continuous wave NMR was used; and either the magnetic field is held constant and the frequency of the electromagnetic radiation (radio waves) is varied, looking for absorptions, or the energy of the radio wave is kept constant and magnetic field is altered.
Today, most NMR spectrometers work in a different fashion, to increase the sensitivity of the technique; the resonances of all the protons are measured simultaneously, and rather than looking for absorptions, we measure the radio frequencies that are emitted by the protons, as they relax back to the ground state after being excited. However, both techniques give us the same information, i.e. the energy gap between the spin up and spin down states, and therefore report on the chemical environment of the protons.
A short, intense pulse of radio energy is applied to the sample, which is absorbed by all of the protons within the sample. After this pulse, the sample emits radio waves, which can be detected:

Notably, it is not as simple as considering simple transitions from high energy to low energy states as emitting detectable signal. Normally, when the protons behave like gyroscopes that are all independent of each other, we cannot detect any net signal from them because they cancel each other out, but by applying a radio frequency pulse of the correct frequency we can cause them to all resonate in a synchronised fashion temporarily. When this happens they generate a signal that can be detected. The signal decays (which we call relaxation) due to both the system returning back to equilibrium (longitudinal relaxation, or T1 relaxation), as the high energy protons return back to the lower energy states, but also as the ‘gyroscopes’ get out of sync with one another, which decays the signal (transverse relaxation, or T2 relaxation).
NMR Spectrometers
NMR spectrometers are expensive pieces of kit to buy and maintain, and therefore most research labs use a centralised facility. NMR spectrometers range in price over the same sort of price range as property; a small bench-top NMR spectrometer with a permanent magnet costs around £60,000, whereas a high field spectrometers cost several millions of pounds!
The strong magnetic field in most NMR and MRI instruments is produced using a superconducting electromagnet; a current is passed through a superconducting wire coil. In order for the wire to be a superconductor, and therefore produce a huge magnetic field, they have to be cooled to very low temperatures, using liquid helium. The actual structure of the electromagnet coil is quite small, and the huge container around the NMR and MRI instruments is just a giant thermos flask full of liquid helium and liquid nitrogen, to keep the magnet at -269 oC. This can be seen below in the photographs taken by Dr Alan Kenwright at Durham University, of an old NMR instrument that has been cut open so that the superconducting magnet (yellow) can be seen inside the cooling compartments, that are filled from the top ports with liquid helium, and liquid nitrogen (outer jacket). Therefore, each NMR magnet has to be filled with liquid nitrogen every week, and with liquid helium every month…. to keep the magnetic coil superconducting.
Running an NMR spectrum of a sample
To prepare a sample for NMR, it needs to be dissolved in solution. If we just dissolved it in a standard solvent, all we would see would be the solvent; it would completely swamp the sample! Normally for NMR, we tend to dissolve approximately 0.01 g of our compound (for example with a molecular mass of 500), in 1 mL of solvent, which is a concentration of around 0.02 mol dm-3. However, the concentration of, say, pure water, is 56 mol dm-3, so if we dissolved the sample in water, there would be nearly 3000 more water protons in the sample tube than the protons in the molecule that we are trying to look at! Therefore we tend to dissolve the sample in solvents that do not contain protons – but these are hard to find! Historically scientists used to dissolve samples in CCl4, but this is not very common these days, since the Montreal Protocol, which led to the phasing out of carbon tetrachloride and other similar ‘CFCs’ that cause ozone depletion. Instead, chemists tend to use deuterated analogues of common lab solvents, such as deuterated chloroform (CDCl3), and heavy water (D2O); the protons are replaced by the deuterium isotope of hydrogen (which has an extra neutron), and therefore it has different magnetic properties to the proton, and does not ‘resonate’ when protons are excited by a specific frequency of radio waves.
How does NMR relate to MRI?
 Have you ever been for an MRI scan? You are probably familiar with images such as that shown on the left, and you may be familiar with a long tube that you have to lie in – well this is just a very strong magnet, exactly the same as the instrumentation used to run an NMR spectrum. MRI stands for magnetic resonance imaging, and uses NMR to create an image; in NMR – we put our sample in an NMR tube, and place it in magnet; this is exactly the same as the MRI magnet, but it is usually the other way around – we don’t tend to want to make patients stand or ‘hang’ vertically! We use NMR to tell us information about the molecules in the sample tube, whereas in the body we are looking predominantly at water, and use a magnetic gradient across the body, so that we can use the NMR information to tell us where the water molecules are, and build up an image. To read more about MRI, click here.
Have you ever been for an MRI scan? You are probably familiar with images such as that shown on the left, and you may be familiar with a long tube that you have to lie in – well this is just a very strong magnet, exactly the same as the instrumentation used to run an NMR spectrum. MRI stands for magnetic resonance imaging, and uses NMR to create an image; in NMR – we put our sample in an NMR tube, and place it in magnet; this is exactly the same as the MRI magnet, but it is usually the other way around – we don’t tend to want to make patients stand or ‘hang’ vertically! We use NMR to tell us information about the molecules in the sample tube, whereas in the body we are looking predominantly at water, and use a magnetic gradient across the body, so that we can use the NMR information to tell us where the water molecules are, and build up an image. To read more about MRI, click here.

This work is licensed under a Creative Commons Attribution 4.0 International License.

