
Soap and Surfactant Chemistry is based on two main concepts:
- HYDROPHOBIC AND HYDROPHILIC NATURE OF SUBSTANCES
If you have ever tried to mix water and oil from the kitchen, you will see that no matter how hard you try, they do not mix.
This is because oil does not “like” water – it is hydrophobic (water-fearing)
Water molecules are polar, meaning they are hydrophilic (water loving)
This explains why oil and water organise themselves into 2 distinct layers, which is what you will eventually see if you mix oil and water.
- OPPOSITE CHARGES ATTRACT AND LIKE CHARGES REPEL
–Positive charge attracts negative charge
-Negative charge attracts positive charge
-Positive charge repels positive charge
-Negative charge repels negative charge
Soap and surfactants work based on these ideas
It is made up of molecules that are both hydrophilic and hydrophobic, making it a surfactant (‘surface-active agent’).
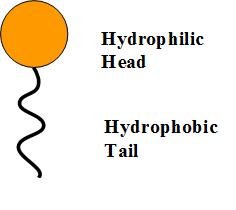
- The head is hydrophilic
- The hydrocarbon tail (made of hydrogen and carbon) is hydrophobic
The heads will stick together, as will the tails. This leads to the formation of micelles.

A MICELLE – the hydrophobic tails arrange themselves in the middle of the structure, with the hydrophilic heads around the outside
How Soap works
Soap likes both water and oil, which makes it a surfactant – it is both hydrophobic and hydrophilic.
When you wash your hands, the soap bridges the hydrophilic water and hydrophobic oil (which contains germs) on your hands.
Germs stick to the layer of oil we all have on our hands.

The soap is attracted to the oil, and encloses it in micelles – this ensures the hydrophilic heads of the soap are touching the water, and the hydrophobic tails are touching the oil.
The foam that appears when washing your hands contains these oily structures, and ends up being washed off your hands.
Because the hydrophilic heads of the micelle are soluble in water, they can be washed away – so when you rinse soap off with water, the soap carries the germ-containing oil with it and this ends up going down the sink!
The soap forming these micelles is also described as the soap forming an ‘emulsion’ – small droplets of oil liquid are suspended in water
Surfactants have a range of uses, involving soap, laundry detergent, shampoo, hair conditioner and fabric softeners. They can be positively charged, negatively charged or neutral – this gives them different properties for their different uses.

This work is licensed under a Creative Commons Attribution 4.0 International License.