 Background
Background
The generation of clean, efficient and environmentally friendly energy is a major challenge for science and engineering. Carbon dioxide levels are 40 % higher than they were in the 19th century, at the beginning of the industrial revolution, and most of the increase has taken place since 1970. During the last 40 years the global energy consumption has accelerated, and the rise in CO2 is largely due to the combustion of fossil fuels. There are growing concerns regarding the increasing greenhouse gas emissions from fossil fuels, as well as the diminishing fuel reserves, and therefore scientists are looking towards fuel cell technology for future power. Fuel cells have a wide range of potential applications, including portable device and transport applications.
A fuel cell, like a battery, is an electrochemical cell, which converts chemical energy into electricity, via a chemical redox reaction. Fuel cells convert the chemical energy associated with the combustion of a fuel gas directly into electrical work, which is much more efficient and environmentally clean than the process of combustion itself. A spontaneous redox reaction produces a potential difference between the two electrodes. The electrode at which the reduction occurs becomes the cathode, which is positively charged, and the oxidation occurs at the anode, which is negatively charged. Electrons flow from anode to cathode.
A fuel cell is different to a battery, in that the fuel and oxidant (the chemicals that react to provide a voltage) can be continuously fed into the system, to react and produce electricity; the cell does not have to be disposed of or re-charged once the fuel runs out, like batteries. As long as the fuel is continually supplied, the cell will continue to operate.
Hydrogen fuel cells use hydrogen and oxygen as the reactants: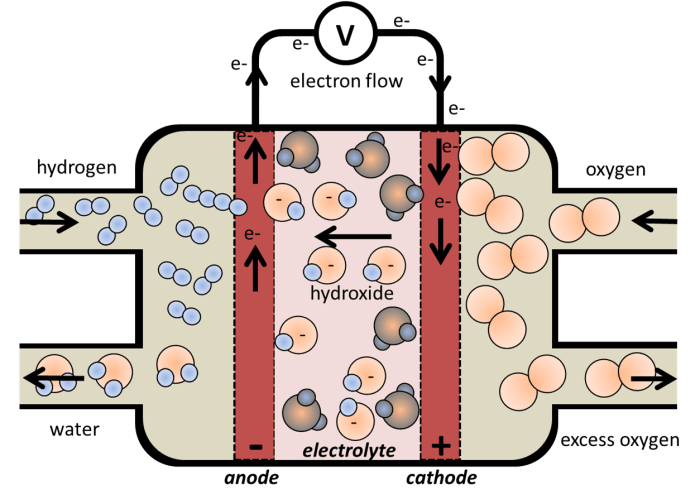
| The alkaline fuel cell:
(-ve electrode) = Anode: H2 + 2 OH– → 2 H2O + 2 e– |
The experiment
A pdf version of this experiment is available here. Please ensure you refer to the safety card. Details for teachers or technicians can be found here.
AIM
To make a battery and a hydrogen fuel cell

YOU WILL NEED:
- 6 small potatoes
- 6 x 2 pence (or 1 pence) pieces
- 6 x galvanised screws/nails
- 7 insulated wires with crocodile clips
- a voltmeter
- an LED
- 2 graphite electrodes (2 pencils work well, sharpened with a knife of both ends to extend the graphite, or 6 x 0.7 mm leads for propelling pencils, taped together per electrode)
- selotape
- Blu tack
- beaker
- 0.4 M KOH (aq)
- 1 x 9 V battery
- plastic knife
- universal indicator paper
PROCEDURE
Part 1 The Potato Battery
Push a penny about half way, into a potato. Push a nail or screw into the potato, about 3 cm away from the penny, so that only 1 cm of the nail remains above the potato surface. Connect a crocodile wire to the penny, and to one side of the voltmeter, and another, to the nail and the other side of the voltmeter, and record the voltage. Now disconnect the voltmeter, and attach the crocodile clips to the LED – does it light? You need to connect the LED the right way around; connect the nail to the negative side of the LED, i.e. the shorter wire, and the copper coin to the positive, longer, wire of the LED. Make up 5 more potato batteries, and connect them together, starting with 2, 3, 4, 5, and all 6, measuring the voltages each time. This activity may need to be done as a class, connecting up with other students’ potato batteries. Take a slice of potato and measure its pH.
Part 2 The Alkaline Hydrogen Fuel Cell
Take the two pencil electrodes, and tape them together, using a piece of Blu tack in between the pencils, to space them, about a pencil width apart. Put 100 mL of 0.4 M KOH(aq) solution into a beaker, and place the electrodes into the beaker.
Electrolyse water to make you hydrogen/oxygen fuel stock: Firstly you need to make your feedstock of hydrogen and oxygen fuel. The simplest way to do this is to hydrolyse the water, using a 9 V battery. Connect a crocodile wire to the top of each pencil, with the clip connected directly to the graphite ‘lead’ of the pencil, and connect each wire to the anode and cathode of the 9 V battery. Observe the electrodes within the water, and leave them connected for 2-3 min. You should see bubbles forming on the graphite electrodes.
Electrolysis:
(+ve electrode) = Anode: 2 OH–(aq) → 1/2 O2(g) + H2O(l) + 2 e–
(-ve electrode) = Cathode: 2 H2O + 2 e– → H2 + 2 OH–(aq)

Make your fuel cell: Disconnect the battery, and connect the electrodes up to the voltmeter, and observe the voltage over 5-10 min. Do this carefully, trying not to disturb the bubbles on the electrode surfaces, as this is your fuel! If you have time, charge up your fuel cell with the battery again, and see if you can light the LED. You will probably need to connect a few cells together. You need to connect the LED the right way around; connect the negative electrode (where you connected the negative end of the battery and the hydrogen was produced) to the negative side of the LED, i.e. the shorter wire.
The alkaline fuel cell:
(-ve electrode) = Anode: H2 + 2 OH– → 2 H2O + 2 e–
(+ve electrode) = Cathode: 1/2 O2 + H2O + 2 e– → 2 OH–
Questions
- The potato battery is made up of two electrodes, and an electrolyte. What is acting as the electrodes, and what is acting as the electrolyte?
- What is the pH of the potato?
- This battery is an example of an electrochemical cell. Chemical energy is converted into electricity, due to a spontaneous redox reaction. The potato contains organic acids, and at the copper electrode (penny), protons from the acid form hydrogen. At the zinc electrode (galvanised screw), Zn oxidises to form Zn2+. Give the half equations and overall equation for this cell.
- What parts of the cell are being ‘used up’ in the chemical reaction, i.e. what is behaving as the fuel within the battery? What will happen when these eventually run out? Are they easy to keep renewing in the system?
- What happens to the voltage as you connect up more potatoes? Can you light the LED?
- For the fuel cell, you first need to electrolyse water to produce hydrogen and oxygen gas. What type of energy is being converted here?
- You should observe the two electrodes bubbling, and one will bubble more than the other. Which bubbles more, the electrode attached to the positive or negative end of the 9 V battery? Write an equation for the reaction that is occurring – why do you produce twice as much gas at one of the electrodes?
- When you disconnect the battery, and observe a voltage, what happens to this voltage over time? Why is this? What is the fuel in this cell?
- What by-product is made when the hydrogen and oxygen react in a redox reaction? Why is this a ‘greener’ process than combustion?
- What are the advantages and disadvantages of hydrogen fuel cells?
Going further
The principles behind fuel cell technology have been known since the work of Christian Friedrich Schönbein and William Robert Grove in the 1830s. Since the development and understanding of these ‘wet-cell’ (liquid electrolyte) batteries, in which the electrolyte is a solution, the development of ceramic (solid) electrolytes began. The electrolyte of a cell conducts positive or negative ions, in order to complete the circuit. We usually think of an electrolyte as being a liquid, in which the ions are free to move, but some solid ceramic materials can also conduct ions, which allows us to eliminate the liquid phase of a fuel cell. This makes for easier construction (leakage of the electrolyte is no longer an issue), and greater chemical and physical stability. In these solid oxide fuel cells, a ceramic electrolyte is used to conduct ions from the cathode to the anode.
In the alkaline fuel cell, the reduction of oxygen produces hydroxide ions, which then react with the hydrogen to oxidise it to form water. Thus, the hydroxide ions have to migrate from one electrode to the other, in an aqueous electrolyte. In a ‘solid-oxide fuel cell’, redox still occurs between hydrogen and oxygen gas; the oxygen reduces at the ceramic surface to form oxide ions (O2-), and these migrate through the ceramic structure towards the anode, to oxidise the hydrogen gas, forming water. Solid-oxide conductors are crystalline inorganic structures, which have available space within the crystal structure that allows oxide ions to move through the lattice at high temperatures.
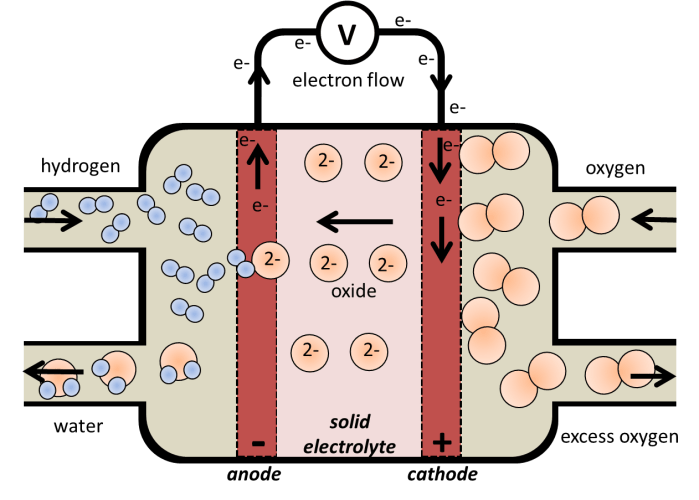
The solid-oxide fuel cell:
(-ve electrode) = Anode: H2 + O2- → H2O + 2 e–
(+ve electrode) = Cathode: 1/2 O2 + 2 e– → O2-
Origami exercise
Download the following Origami Fuel Cell, and print onto A4 paper, preferably in colour, to make a 3D cube that shows alkaline and solid-oxide fuel cells:
This resource has been adapted from a teaching resource by Professor Peter Slater.
In the research lab
Researchers working with Professor Peter Slater, at the University of Birmingham, are developing a range of new ceramic materials for the electrode and electrolyte materials for solid-oxide fuel cells. Current work involves the development of new ceramics that can reduce the operating temperatures of solid-oxide fuel cells, to extend their operating times, as current zirconium-oxide type structures only conduct oxide ions well in the 800-1000 oC temperature range.
Publications of this work

Abstract above reprinted with permission, copyright (2017) American Chemical Society http://pubs.acs.org/doi/abs/10.1021/jacs.5b13409
Files for download
pdf files:
Fuel cell experiment details for students
Fuel cell experiment details for technicians and teachers
Fuel cell experiment safety card
Fuel cell experiment guide for teachers
editable files:
Fuel cell experiment details for students word document

This work is licensed under a Creative Commons Attribution 4.0 International License.

