 Background
Background
Sensors are one of the many areas of technology that rely on the development of new nanomaterials, and their manufacturing techniques. Information about our health can be extracted from biological material in our saliva, blood, urine and sweat, in order to detect the onset of disease. When a doctor carries out tests to detect biological material, they often need to take blood samples, filling up several tubes, and send them off to a centralised laboratory. Technicians then take the samples, perform several analytical tests on the material, and send it back to the doctor. If the doctor only needed a drop of blood and could test for disease while you wait in surgery, the whole process is a lot quicker, and a lot less labour-intensive. The sooner a disease is diagnosed, the more likely it can be managed or cured. Many of these ‘disease markers’ include proteins and DNA, which are tens of nanometers in size, i.e. thousands of times smaller than the diameter of a human hair, and hence interact selectively with materials on the nanoscale.

In order to detect disease from very low concentrations of biological material, it is beneficial to shrink down all the processes necessary to detect the material onto a tiny ‘chip’, to create a ‘lab-on-a-chip‘ device. Researchers at the University of Birmingham are developing functional nanosurfaces on gold-plated glass chips to detect prostate cancer. Scientists are working with Alta Innovations, and the test could soon be commercially available.
Glass slides are coated in gold, as gold-sulfur chemistry can be exploited to create functionalised surfaces with ease. The surface is then coated in molecules that contain sulfur, using a protein that tends to be found in the blood when someone has prostate cancer, to create a template on the surface. This is explained in the BBC video press-release below:
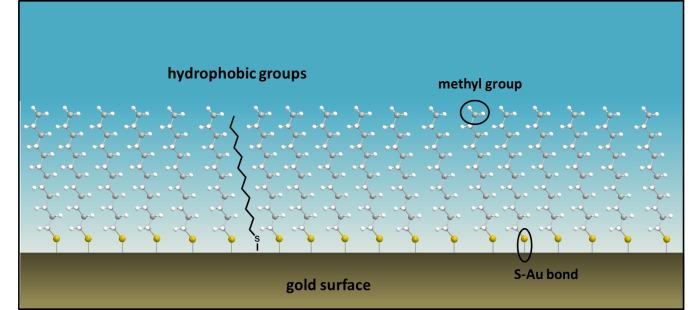
Many functional nanosurfaces are created using gold surfaces, on which chemists create a SAM, i.e. a self-assembled monolayer. This is an assembly of organic molecules that form spontaneously on a metal surface. Often long chain molecules, with a thiol (-SH) group at the end are dissolved in ethanol and deposited onto a gold surface. Sulfur binds to gold immediately and strongly, and once the surface is washed, an ordered, single layer of organic molecules (a monolayer) is left behind. This layer determines the surface chemistry now on the gold, and is only a few nanometers in height:
Experiment
A pdf version of this experiment is available here. Please ensure you refer to the safety card. Details for teachers or technicians can be found here.
A single layer of molecules can be attached to a metal surface to form a self-assembled monolayer (SAM), which alters the properties of the surface. In this experiment you will make a silver surface on a glass slide, and then create a single layer of molecules attached to the surface, called a monolayer.
AIM
To make a self-assembled monolayer on a silver surface, and to see how this nanoscale layer affects the hydrophobicity of the surface. (Silver is used as it is much easier and safer to deposit silver onto glass than gold. Sulfur binds to silver also, and similar SAMs are formed).
YOU WILL NEED
- (Tollen’s solution) -This MUST be made in situ by staff and dispose of within 30 min down sink with lots of water – there is a risk of forming explosive silver fulminate
- (0.3 M glucose (aq) solution)
- (glass microscope slides)
- petri dish / watch glass/ large beaker, big enough to fit slide in
- 0.1 mM ethanolic dodecanethiol (DDT) solution
- paper towels
- ethanol
- deionised water
- tweezers
- plastic pipettes
The items in brackets are only needed if students are going to silver their own slides. Alternatively, this experiment can be done with pre-silvered glass slides.
PROCEDURE 1 – making your own silver-plated slides
If your glass slide is fresh out of a new packet, use it as is, being careful to handle it by the edge so as not to get grease on the surface. Otherwise, wash your slide with soap and water, and dry with a paper towel. Wash with ethanol, and dry.
Place 2 glass slides into separate petri dishes/ large beakers/ onto watch glasses, so that they can lie flat. Using a plastic pipette, drop ~4 drops of glucose solution onto each glass slide, followed by ~12 drops of the Tollens’ solution, and agitate for 3-5 min – You should see a silver mirror forming on the glass.
Pick the slide up with tweezers, and carefully wash off the solution with deionised water, followed by ethanol, and place onto a paper towel (silver side up), to dry.
Wash out your petri dishes, and wash all the Tollens’ waste down the sink with plenty of water.
PROCEDURE 2 – forming the SAM on the silvered slide
If you have made your own silver-plated glass slide, dry your petri dishes with a paper towel, and place one slide back into a petri dish. This experiment can also be performed with slides that are already silver-plated for you. In this case, take a provided silvered slide, and place it into a petri dish, and another onto a paper towel. Label this petri dish ‘DDT on silver’, and label the paper towel on which the other slide sits, as ‘bare silver’.
Take the ‘DDT on silver’-labelled slide, and add ~10 drops of ethanolic dodecanethiol (DDT) solution onto the surface. Agitate to cover the slide, and leave for 5 min. Pick up the slide with tweezers, and wash it thoroughly with ethanol, leaving it to dry on a paper towel.
Now test the surfaces for their hydrophobicity. To do this, add a few drops of deionised water onto each silver slide, and observe what happens.

Questions
- Can you see the single nanoscale layer of molecules that are deposited on the surface of the silver? Do the two surfaces look any different?
- When you drop water onto each slide, do they behave differently?
- Which surface likes water, and which surface repels water?
- The SAM (self-assembled monolayer) of dodecanethiol has a terminal -CH3 group on its surface, which is hydrophobic. Why do you think that water prefers polar surfaces, such as clean metal surfaces that have negative surface charge, rather than a non-polar monolayer of organic molecules?
- In the research lab chemists build SAMs on gold surfaces. Why do you think this is? (The bond dissociation energies of Au-S and Ag-S at 298 K are 253 kJ mol-1 and 217 kJ mol-1 respectively.)
- The image below shows SAMs on three gold surfaces. Describe the difference in the droplet shapes, and explain why you think this is happening?


publications of work using SAMs for Sensing Applications
To access the above paper, click here.
Files for download
pdf files:
Gold sensing experiment guide for students
Gold sensing experiment details for technicians
Gold sensing experiment safety card
Gold sensing experiment guide for teachers
editable files:
Gold sensing experiment guide for students word document

This work is licensed under a Creative Commons Attribution 4.0 International License.
