Download a printable version of this document here
Catalysts are useful tools in organic synthesis as they speed up a chemical reaction without being changed themselves, meaning they can be recovered at the end of the experiment and used again and again.
Catalysts speed up reactions by offering alternative reaction pathways. The energy needed for a reaction to occur is called the activation energy. If this is very high, the reaction requires high temperatures and can be very slow. Take a look at the energy diagram here and think of it as the reaction needing to climb a very steep hill. This is very hard work and the reaction needs to use more energy to get over the top and reach the other side. This is slow work and it can be quite expensive to heat the system sufficiently to give it enough energy.
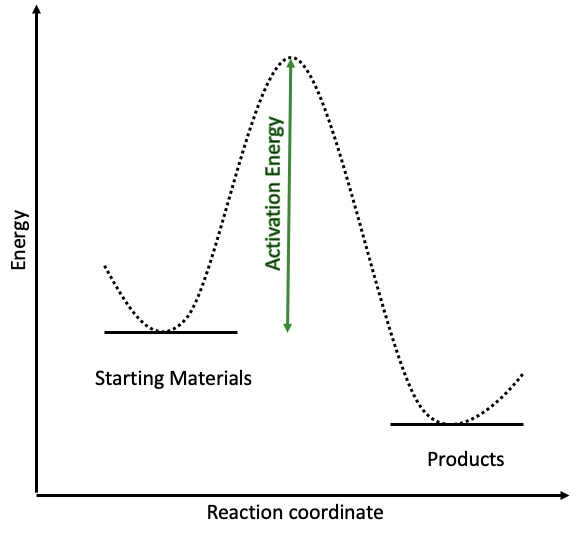
However, if we add a catalyst to this mixture, we can reduce the activation energy needed to complete the reaction.
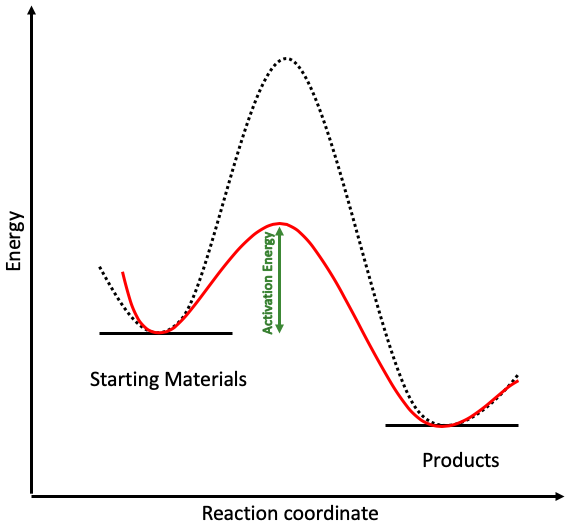
The catalysed reaction in red has a much lower activation energy than the uncatalysed reaction. Going back to the hill from earlier, adding a catalyst into the mix makes the hike much easier, reducing the height of the hill and requiring less energy. This is great news for chemists because their reactions can happen much quicker under less severe conditions.
The best part is, catalysts are not directly involved in the reaction. As a result, you can reform/recover a catalyst at the end of an experiment, meaning we can keep using them for many different experiments.
Transition metals
Transition metals and their metal complexes make up the vast majority of catalysts used in scientific research and industry. This is for 2 reasons;
- They have variable oxidation states
- They allow substrates to adsorb onto their surface, thereby activating them.
A transition metal can either exist as a solid (with a surface to adsorb too) or as a free metal complex in solution. A solid catalyst that catalyses a reaction of either a liquid or gas that adsorbs to the surface is known as a heterogenous catalyst. An example of this would be the iron surface (solid) used in the Haber process to catalyse the formation of ammonia (NH3).
A catalyst that is in the same state as the reagents it is catalysing is known as a homogenous catalyst. Any catalytic reaction involving a metal complex in solution catalysing a reagent which is also in solution could be an example of homogenous catalysis. A specific example is the use of Wilkinson’s catalyst (PPh3)3RhCl for the catalysis of alkene hydrogenation see below.
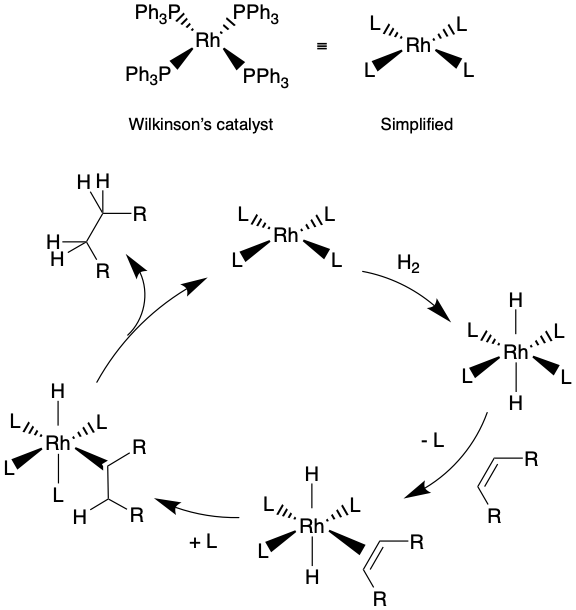
This type of reaction scheme; a catalytic cycle, is a commonly used mechanistic diagram to explain how a metal complex evolves through the course of a reaction, but which ultimately returns to the same configuration when the product is produced. Remember to be a true catalyst, the catalyst must be reformed as part of the reaction!
Not all catalysts however require the presence of a transition metal centre. Synthetic molecules which act as catalysts without the presence of a transition metal are known as organocatalysts. An example of this is DMAP which is commonly used as a nucleophilic catalyst (see example below). The DMAP attacks the starting material (acetic anhydride) making it more reactive and therefore more susceptible to nucleophilic attack via the alcohol (ROH). Once the alcohol attacks the now activated carbonyl, the DMAP is released (due to being a good leaving group) and thus the DMAP is regenerated and ester product formed.

Metal complexes which contain both a transition metal centre and organic molecule ligands surrounding the meal are also termed organometallic catalysts. Wilkinson’s catalyst described above could be described as an organometallic catalysts.
Is that a catalyst?
Some reagents are sometimes mistakenly labelled as catalysts. An example of this is AlCl₃ which is used in Friedal-Crafts acylation. learn more on this chemBAM webpage; Is that a catalyst – Friedal-Crafts acylation.
Learn more about designing sustainable synthesis here.
Go back and learn more about the chemistry of catalysis here.

This work is licensed under a Creative Commons Attribution 4.0 International License.